A lot has changed since chemists pushed into the 20th century, chasing ever-better solvents to feed new markets. Glycol ethers started to show up in labs and plants when people looked for alternatives that offered a balance of solvency and lower toxicity. Dipropylene glycol butyl ether (DPGBE) took its spot on the scene thanks to researchers hunting for smarter molecules—ones that could handle tough dirt in cleaners but dodge the hazardous reputation of their older cousins. Paint makers, formulators, and floor-finish chemists have watched this rise closely. With government bans closing doors for some glycols, DPGBE has gradually filled gaps left by older chemicals banned for environmental or toxicity reasons.
DPGBE hasn't been around as long as ammonia or turpentine, but it's managed to carve out a space among glycol ethers as a dependable go-to for tough cleaning jobs, paint strippers, inks, and hydraulic fluids. People in the know recognize it by several other names on SDS sheets—“Butoxy DPG” or “Propylene glycol butyl ether,” depending on the supplier. It stands out among solvents for having lower odor and softer bite, letting companies make products for indoor use that don't require hazmat suits for routine jobs. The molecular structure tacks together two propylene glycol units with a butyl tail, giving it an edge for blending into water-based or oil-based formulas. Its spot in formulations often comes down to the simple fact: it works deep, doesn't evaporate too quickly, and rarely causes skin burns or headaches if used right.
This clear, nearly odorless liquid slides under most noses unnoticed unless someone’s deliberately sniffing for it. DPGBE has a boiling point above 230°C, which means even the hottest summer shop won’t drive it out of solution in a hurry. Viscosity comes in just thick enough to feel slick but flows easily when tipped, reminiscent of light syrup. Technicians appreciate its miscibility with water and many organic solvents. It quietly cuts through greases, inks, and latex, thanks to a midrange polarity that tackles both polar and non-polar substances. This versatility keeps it in both household brands and industrial applications.
On the label, DPGBE turns up under CAS number 29911-28-2. Purity runs above 98%, with water content kept below 0.1% in quality grades heading to sensitive electronics or coatings. Drums and totes carry the standard GHS labels—warnings for eye and skin sensitivity, but the pictograms steer clear of the most serious hazards. Producers list flash point near 100°C, with specific gravity hovering around 0.95 g/cm³. These specs reassure health and safety officers—spills are less likely to catch fire than with lower-boiling alternatives, and the low evaporation rate means less chemical lost to air.
DPGBE usually comes from a condensation process between butanol and propylene oxide. Industrial reactors mix these under controlled heat and pressure, coaxing a reaction through an alkali catalyst. Water, sodium hydroxide, propylene oxide, and butanol all find their place in this stage. Afterwards, the job goes to purification towers, where distillation removes unreacted butanol and other glycol ether fractions. What’s left gets filtered, scrubbed, and stored in steel tanks ready for shipment. Some facilities tune the recipe for different product lines, adjusting the propylene oxide ratio or running longer purification cycles to guarantee top-end purity for pharmaceutical and electronic applications.
In everyday use, DPGBE acts as a relatively stable solvent but will break down under strong acids or bases. Given enough heat or sunlight, the ether linkages eventually snap and start giving off smaller alcohols and aldehydes. On the production side, chemical engineers sometimes tweak DPGBE for specialty polymers or surfactant applications, adding reactive end-groups that help bind it into chains or emulsions. These branches let the molecule contribute to formulations in paints or adhesives that demand stronger performance. Even with all its resilience, DPGBE remains human-friendly so long as chemists avoid pairing it with potent oxidizers or mixing with reactive halides.
Anyone flipping through supplier catalogs or managing a warehouse might find DPGBE lurking under several aliases—“Butoxy DPG,” “1-Butoxy-2-propanol,” “Dowanol DPnB,” or “Arcosolv DPnB.” Brands create their own semi-trademarked versions, branding those for use in paint strippers or household cleaners. Staying on top of these names keeps purchase and process documents from getting crossed up, especially when regulatory teams review inventory or prep compliance reports.
From a hands-on standpoint, working with DPGBE feels a bit less dicey than tackling some other glycol ethers or heavy-duty solvents. With gloves and goggles, labs and production plants can handle routine transfers, cleanups, and blendings without breaking stride. OSHA and EU standards mark it as an irritant but steer clear of stronger danger classifications, though workers still stick to good habits—ventilation, proper storage in sealed drums, and routine air quality monitoring. In case of overspills, water and soap take care of minor mess, but bigger jobs get absorbent pads. Exposure limits reflect cautious optimism, pegged higher than many competing solvents. Still, training and regular health checks remain part of industrial culture.
Factories keep DPGBE on hand for jobs modern society can’t easily replace—making water-borne coatings roll on smoothly, thinning latex paints so rollers glide, or powering through hardened grime in commercial kitchens. The printing industry benefits, loading DPGBE into ink blends to ensure the right drying times and sharp edges on packaging. Auto shops deal with brake fluid blends containing DPGBE for its lubricating effect and low volatility. Homeowners unknowingly work with it every time they apply modern multipurpose cleaners or window sprays. Hospitals and schools lean on cleaning supplies built around this molecule, appreciating the reduced harshness on skin or airways during repeated applications.
In research circles, teams chase ways to modify DPGBE structure for greener formulations or expand the range of plastics and adhesives it can support. Universities and corporate labs test new additive packages that help it mop up heavier contaminants in wastewater or function as a carrier for agricultural sprays. Novel processes aim to cut production energy by tailoring catalysts that work at lower temperatures or by using recycled feedstocks. Reports note some success in pushing DPGBE into performance roles for specialty inks, high-durability floor coats, and as a substrate for designer surfactants—each step opening new product classes that demand solvent power and flexibility.
Toxicologists have spent years checking DPGBE’s profile for hidden risks. Animal studies suggest low acute toxicity, so brief exposures rarely cause lasting harm. Human cases, mostly accidental spills or splashes, produce skin redness or mild irritation rather than anything more severe. Chronic exposure tests, including long-term inhalation studies in rodents, found few signs of organ toxicity or cancer links at workplace concentrations. Regulatory bodies review this growing literature to set exposure thresholds, giving factories the confidence to keep using the chemical in regulated spaces. Research gaps remain around long-tail metabolites and water treatment breakdown products, but so far, signs point to a chemical that, with ordinary caution, won’t upend worker health or public safety.
Looking out over the next decade, DPGBE could see even heavier use as countries retire old-school solvents and industries demand greener, less hazardous alternatives. Waterborne coatings keep growing as global regulations squeeze volatile organics from paints, driving DPGBE into new markets with every regulation passed. Polymer scientists keep probing the molecule’s stability and reactivity for next-gen bioplastics and compostable films. Some companies bank on advances in bio-based production pathways, hoping to switch from petrochemical feedstocks to plant-derived propylene oxide. These changes could further shrink the environmental footprint, keeping DPGBE relevant long after competitors face stricter controls or phase-outs. If researchers can close the toxicity data gaps and production lines can shift to sustainable sources, DPGBE stands ready to outlast many fads in the chemical world.
Dipropylene glycol butyl ether pops up in almost every cleaning aisle you wander. Look at any bottle of degreaser or all-purpose spray, and odds are you'll spot this chemical in tiny text. Surfaces covered in grime, greasy kitchen counters, or even stains on tile get trickier to clean when the active ingredients can’t cut through old gunk. This is where dipropylene glycol butyl ether steps in: it acts as a solvent, helping dissolve greasy messes and oily films. I’ve noticed the difference at home—cleaners with it seem to leave fewer streaks and handle sticky residue with less scrubbing. Manufacturers use it partly because it blends well with both water and oil, which means better performance in a larger range of products.
You don’t have to spend long painting walls or furniture to realize how much a smooth finish matters. If drying time is too quick, paint goes tacky and blotchy. Dipropylene glycol butyl ether slows down evaporation just enough, so you wind up with a smoother surface. It works well in inks and lacquers too, giving artists and manufacturers more control. I remember using water-based paints that went on unevenly—those without good co-solvents just wouldn’t lay down flat. This chemical actually helps the pigments and dyes float evenly, which keeps the colors looking sharp without brush marks.
People often complain about overpowering smells from using industrial or household chemicals. Old-school solvents easily stunk up a house and often came with hefty health headaches. What sets dipropylene glycol butyl ether apart is the low odor compared to others in its class. That means it keeps the air more breathable, which matters a lot to anyone with asthma or allergies. Safer handling also stems from its lower toxicity, and that’s no small thing. Not long ago, heavy-duty cleaners left my hands irritated within minutes, but products with this glycol ether seem far gentler, based on personal experience and industry feedback.
Cement factories, food plants, and even textile mills use dipropylene glycol butyl ether since it helps both as a cleaner and a processing aid. In textile work, it softens tough stains and makes dyes sink deeper into fibers. Food processing machinery needs serious cleaning that won’t leave dangerous residues, and this solvent helps get equipment sparkling without posing much risk. It’s become a staple in leather treatments and certain pesticides, again for its balance of strength and low toxicity.
Workers deserve safer products that still deliver solid results. I’ve chatted with maintenance crews at schools and restaurants, and many trust cleaning agents with this chemical for its muscle and milder impact compared to old petroleum-based choices.
Dipropylene glycol butyl ether makes a big difference from kitchen counter sprays to factory floor cleaners. While it generally ranks low in immediate risks, overuse or improper disposal can eventually affect waterways and indoor air. Recycling and treatment plants need upgrades to handle these residues better. Companies should give more info on labels so that folks actually know what’s in their sprayers or paints. Opting for smaller amounts in formulas, or using alternative solvents when possible, keeps exposure in check. The best move will always be honest communication about what these chemicals do and how to use them with care.
Dipropylene glycol butyl ether pops up in a lot of cleaning products, paints, and even some personal care formulas. It's a solvent, which means manufacturers put it in their goods to help mix stuff together or make things spread and dry the right way.
Most folks interact with this chemical when they wipe down windows or mop floors. Regulators like the US Environmental Protection Agency and the European Chemicals Agency check out substances like this before they land on store shelves. Their reports don’t ring immediate alarm bells for most uses, especially when folks follow label directions. Workers inside factories using barrels of the stuff see stricter rules, which just makes sense since their exposure is a lot heavier than cleaning a mirror at home.
People get nervous about chemicals with names this long, and who could blame them? The average shopper looks at a label packed with unfamiliar terms, and it’s natural to worry about long-term health effects. Science tells us dipropylene glycol butyl ether doesn’t mess with your body much if you just splash some on your skin or breathe a little in while cleaning. You won't see rashes or lung issues for most people using it the regular way. If you swallow a whole bottle or splash it in your eyes, you’re going to need medical help fast—just like with most cleaners.
The chemical doesn’t build up much in the environment. Water treatment plants break it down pretty quickly, so it won’t stick around in rivers or soil for years. Fish and plants in local waterways don’t drop dead after a spill, unless someone dumps massive amounts at once, which hardly ever happens outside of a big industrial accident.
At home, people mostly deal with small doses—one splash in a diluted cleaner or a bit in a spray bottle. Only a few jobs bring workers face-to-face with vats of it. Factories rely on gloves, goggles, and proper ventilation. These rules keep air in workspaces clear and help workers avoid skin irritation. At my first job mixing big batches of chemical cleaners, I learned fast to treat protective gear as non-negotiable. Coworkers who’d skip gloves just once wound up with red, itchy skin. Management made changes to reduce exposure, and nobody missed the rashes.
Not everyone wants to deal with any amount of man-made solvents. Some brands respond by selling vinegar- or plant-based cleaners. Even big box stores now carry these "greener" choices. They often work just fine for light jobs, but scrubbing up tough stains sometimes means reaching for the old standbys.
Folks looking for peace of mind can read product labels, check safety data sheets, and look up information through official sources. If something stings your skin or burns your nose, swapping it for something gentler never hurts. Proper ventilation—like opening a window—goes a long way toward keeping your lungs happy while you clean. Good gloves shield the skin. Trusting science-backed recommendations over social media hearsay keeps things in perspective.
Industry keeps exploring safer chemicals. Regulation helps push that along. For now, dipropylene glycol butyl ether works for most people when treated with a bit of respect. Taking simple precautions at home and work keeps this solvent from becoming a problem.
Dipropylene glycol butyl ether grabs attention for its role in cleaning products, paints, inks, and coatings. The name might sound like a mouthful, but at its core, this is a solvent with some handy traits that set it apart. It flows like a typical clear liquid. Pick up a bottle of it, and you’ll notice there’s no color, and it doesn’t hit you with a harsh odor, so users don’t flinch when they open a jug.
Let’s talk numbers. This stuff weighs about as much as water, with a density sitting at around 0.95 g/cm³. It mixes with water very easily, so you’re not left with clumps or bubbling, just a smooth blend, which matters if you’re someone working to whip up a batch of industrial cleaning solutions. If you splash a bit, it evaporates at a medium pace – not lightning-fast like acetone, but nowhere near as slow as motor oil.
The boiling point tells another story. You can heat dipropylene glycol butyl ether past 230°C before it starts to boil. That gives it a solid range in processes where temperature can swing without causing headaches. Pour some in your hand and you won’t see or feel “greasy” residue; it’s got a kind of “slip-through-you-fingers” feel, meaning operators don’t have to scrub after spills.
Look under the hood. Chemically, this solvent belongs to a group called glycol ethers, which means it pulls double duty blending both water and oils. That opens the door to cleaning products that tackle gritty dirt as well as greasy marks. Paint manufacturers rely on this, too—they want colors to go on evenly, not in blobs or streaks.
Dipropylene glycol butyl ether laughs in the face of acids and bases. It doesn’t break down or fizz up if you combine it with bleach, ammonia, or vinegar – I’ve worked with enough test batches to know that this resilience means less wasted product and fewer ruined batches. Even strong oxidizers don’t bother it unless you really crank up the heat. This offers peace of mind for people blending multiple strong ingredients in industrial settings.
These properties add up to more than a list on a safety sheet. At home, in the garage, or on a factory floor, workers don’t always have time to double-check if mixing something will wreck the blend or gum up the works. The chemical stability of this solvent means less risk of accidents and more confidence when experimenting or scaling up a recipe.
People with sensitive skin might run into some problems if they get dipropylene glycol butyl ether on their hands often enough. Gloves take care of that, but it speaks to a wider issue. Safety training sometimes gets overlooked because the liquid looks harmless. That’s a bad habit. The safety data is clear: eye irritation and skin dryness are real concerns. Ventilation and gloves aren’t optional, they’re just smart.
I’ve been in enough labs and janitor’s closets to see this solvent earn its keep. Its moderate volatility, solid blending skills, and chemical toughness keep it in use. Cleaner manufacturers should keep pushing for clearer, easier-to-read warning labels. Paint companies have started exploring greener alternatives, but for now, dipropylene glycol butyl ether fills a spot that’s tough to replace. If anyone is hunting for a single “do-it-all” solvent, this one pulls more than its weight—just remember the gloves.
Anyone who’s dealt with chemicals knows every label carries a story. Some stories spell out danger in bold letters, others whisper about subtle risks. Dipropylene Glycol Butyl Ether fits into that second category. You won’t catch it exploding at a moment’s notice or giving off clouds of toxic smoke, but it's still got quirks you can’t ignore. In my earlier years at a small industrial shop, I learned that treating every drum and container with respect saves you more time, money, and drama than cutting corners ever will.
I’ve seen warehouses set aside corner spots for all sorts of substances, and this one asks for the basics: cool, dry spaces away from direct sunlight and any open flames. Humid corners spell trouble. Moisture doesn't help the integrity of containers, and it lets rust sneak in. Since this chemical enjoys staying in liquid form, brush away any thoughts of letting it freeze or bake in the heat. Each time you walk through a warehouse, check if any air vents blow hot air onto storage shelves. Keeping drums off concrete floors using pallets helps keep them away from temperature swings and ground moisture.
Forget laziness with labels. Every container should wear its identity loud and clear, without faded ink or torn stickers. I’ve had visits from safety inspectors who’ve doubled back just because a label looked suspicious or half-missing. Everyone on your team benefits from knowing exactly what's inside, including emergency crews if a spill ever occurs.
A closed-off room never did any favors for chemical vapors. I’ve worked places where stale air clings around storage areas, and once, we dealt with headaches because no one bothered to prop open a door or check the fans. Install ventilation if your space doesn’t already have it. Don’t stuff shelves right to the ceiling or block grates and fans with boxes. Your nose shouldn’t register anything too strong if the airflow works as it should.
People talk a big game about personal protective equipment, but I’ve watched plenty of folks try to handle chemicals bare-handed. Washing your hands afterwards doesn't help much if a liquid seeps through your skin. Gloves resistant to chemicals and long sleeves block splashes. Eye protection isn't just for pouring; it matters even while moving containers to avoid nasty surprises from leaks. Once, a friend of mine ignored goggles and wound up getting solution in his eyes. Even “mild” chemicals still mess up your day.
Keep absorbent pads and spill kits nearby. I remember the panic the first time we knocked over a half-full jug without anything on hand to clean it up. Now, we make sure every room has plenty of material for soaking up spills, and buckets for quick disposal. Clean up straight away; don't let puddles linger on the floor.
Reading through manuals only gets you so far. Every new worker should see a demonstration. Walking through the common mistakes and watching someone handle storage or a spill leaves a stronger impression than checklists ever could. Add regular reviews — people forget, and habits slip. Post reminders or hang laminated sheets with simple steps where everyone can see them.
Taking these simple steps with dipropylene glycol butyl ether rewards you twice: first, fewer accidents or health scares, and second, standards that protect your coworkers, the environment, and your budget without asking for over-the-top investments. Safe storage and handling aren't tough. They just call for sticking to good habits every single day.
Every time someone mops a floor in an office or sprays a streak-free cleaner on a kitchen counter, there’s a good chance Dipropylene Glycol Butyl Ether is in the mix. This solvent shows up in everything from industrial cleaners to household soaps. It brings real convenience—dissolving grease, spreading fragrances, making products last. For a long time, few folks outside the chemical plants spent much time worrying about it. The story is starting to change, and for good reason.
Chemicals don’t stay where they start. In my own neighborhood, it’s no surprise to see storm drains foaming white after a hard rain. Once a barrel of cleaner tips over at a warehouse or a washing machine empties into city pipes, runoff becomes part of the water cycle—and Dipropylene Glycol Butyl Ether can ride that river all the way to the sea. Studies have shown it sticks around long enough to slip through standard water treatment. I’ve read state environmental agencies call it “readily biodegradable,” but that tag doesn’t mean it disappears without a trace. Breakdowns vary by environment and time. Too often, faster degradation outside the lab turns into slow fading in the real streams and lakes.
Small fish, aquatic insects, and bottom feeders carry the real burden. Even if this solvent poses less acute toxicity compared to harsher chemicals, chronic low exposure chips away at animal health. I’ve fished with older neighbors who remember catching frogs by the handful; now, those puddles smell faintly of perfumed soap, and native insects are almost gone. Research from Europe has flagged glycol ethers for their links to changes in amphibian development and even minor disruptions in fish reproductive cycles.
Human health rarely feels the blunt edge, but we all drink and bathe in water that travels these channels. If you talk to people who monitor municipal wells, they’ll say regulations lag behind science, making the picture murkier for years before action starts. What’s more, many cleaning and personal care products get rinsed or poured away without a thought about where their contents wind up.
Anyone living near a chemical plant knows the air sometimes carries a bite. Dipropylene Glycol Butyl Ether evaporates less aggressively than old-school solvents, but emissions do happen. Workers report headaches or nausea if ventilation lacks punch, and the broader community sometimes reports a sweet, odd odor. Studies in the US and Europe show solvent emissions, even the "safer" types, add up across thousands of facilities.
Change rarely happens because one person swaps out a cleaner, but pressure grows when city councils and companies start reading the science. Some big cleaning companies now disclose every ingredient, so consumers and facility managers can pick products without glycol ethers. Cities can set rules on what flows out of factories, demanding monitoring and cleaner disposal. I’ve seen restaurants shift to vinegar-based or plant-based solvents after local groups brought up river contamination. Public demand pushes stores to stock less toxic options, but this only works with clear, readable labels folks can trust.
Every person who stops to read the back of a spray bottle becomes part of the conversation. The solutions don’t always cost more or work worse; sometimes they just require a shift in habit and a demand for accountability. As more eyes open to what rides through our drains, more voices will ask why we choose one solvent over another—and more rivers could run a little cleaner for all of us.
Walk through any big-box store and most shelves are lined with a dizzying display of brightly colored bottles—cleaners for surfaces, floors, windows, laundry. At first glance, people might see nothing but marketing hype and scents, but a closer look at the ingredients tells a different story. One name that tends to show up where cleaning power is needed is dipropylene glycol butyl ether, often tucked away among chemical listings. It rarely gets much attention, yet it plays a bigger part than most realize.
Dipropylene glycol butyl ether steps up where stubborn stains, greasy residue, and sticky spills push most simple compounds to their limits. It acts like a bridge, able to dissolve both water-soluble and oily substances. In my own home, facing everything from greasy stovetops to a neglected grill, only solutions containing this ingredient seemed to cut through that mix of grime. Without it, there’s a lot more time spent scrubbing, relying on elbow grease over science.
Manufacturers appreciate how well it blends into both water-based and solvent-based solutions. Glass cleaners with streak-free claims often depend on this compound because it lifts away oils but doesn’t leave behind hazy residue. It finds its way into carpet cleaners, degreasers, and even heavy-duty floor cleaners for restaurants and hospitals. Not flashy, just quietly reliable.
There’s a second life for dipropylene glycol butyl ether outside the janitor’s cart. Paints, coatings, printer inks, and even agricultural sprays rely on stable mixing and smooth spreading. Anyone who’s worked with cheap paint knows how a streaky coat turns a simple project into a headache. Adding this solvent helps pigment and base flow together, giving better coverage and cleaner brushstrokes.
Cosmetics aren’t immune to its benefits, either. Lotions or creams with a lush, even feel often turn to glycol ethers to carry both water and oil components for a smoother experience. It’s odd to think a single chemical can belong in both a bottle of glass cleaner and a fancy moisturizer, yet the overlap usually points to its flexible nature—not dangerous, provided it’s used correctly.
No one feels excited about seeing long chemical names on a label. Rightfully so, since people have learned that too many industrial additives can pile up health concerns—especially for workers who handle them all day. Research on dipropylene glycol butyl ether doesn’t trigger the same alarm as heavier-duty solvents like toluene or xylene, but it’s not risk-free. Prolonged exposure to vapor or skin contact invites trouble: eye irritation, headaches, or worse when unprotected.
Regulation tends to keep workplace exposure at safer levels, at least in countries paying attention to worker rights. Better ventilation, gloves, and protective clothing go a long way. At home, the exposure risk drops since concentrations run much lower and most people use cleaners with windows open or in bursts rather than all day.
Manufacturers have a responsibility not just for cleaning power, but also for worker safety and environmental persistence. Safer alternatives keep grabbing headlines, and consumer pushback against harsh chemicals keeps companies searching for ways to drop risk while holding on to results. Finding plant-based solvents, boosting transparency, or even improving packaging to reduce waste all take pressure off chemicals like dipropylene glycol butyl ether without forcing everyone back to hours of scrubbing by hand.
People deserve both clean homes and honest labels. Dipropylene glycol butyl ether might not be a household name, but it shapes our daily routines more than most realize.
Cleaners, paints, inks—just about any household or workplace with shiny floors or bright-colored packaging has likely used dipropylene glycol butyl ether, or DPGME for short. As more folks read labels, anything with a long name starts to make people uneasy. And after seeing plenty of scary news about chemicals in food and air, regular people begin to wonder: is this one alright, or just another ingredient waiting for a ban?
Plenty of government agencies have taken a look at DPGME. The Agency for Toxic Substances and Disease Registry points out that this chemical doesn’t build up in the body or stick around the environment for long, which is already some relief. Most people working with DPGME in factories or warehouses wind up inhaling it more than touching it, but studies show pretty mild effects—eye and throat irritation come up most. People spilling it on their skin day in, day out, might start getting dryness or a rash, but usually only at higher exposures than you get cleaning a kitchen.
The Environmental Protection Agency sets some pretty specific rules for how DPGME gets handled in workplaces or added into cleaning products. Anyone worried about food or drink, it’s not allowed as a food additive in the US, and manufacturers say it should stay out of anything eaten or drunk. Plus, research hasn’t shown it to cause cancer or mess with hormones when used as intended.
Experience running an old print shop taught me that exposure can really ramp up in tightly closed rooms with poor air flow. Back in the day, we’d mop floors using solutions that left behind a sharp, sweet smell—turns out that was DPGME. Working without gloves or fans, a bad headache after a shift was something lots of us took for granted. Once we started opening windows, tried low-odor cleaners, and wore gloves for a change, the headaches disappeared.
At home, folks hardly touch DPGME for more than a few minutes scrubbing a bathroom or washing windows. No surprise allergies or breathing trouble after normal cleaning projects, based on conversations with friends using the same stuff I did at work. Most people worry about what happens if kids or pets come into contact. Swallowing a bottle would mean a trip to the ER, but same goes for just about any household cleaning product under the kitchen sink.
Every chemical has its breaking point. Filling a sink and dipping bare hands in DPGME-laced soap all day would probably give just about anyone a rash. Inhaling concentrated fumes in a tiny, closed room could irritate eyes or noses. But millions safely wipe it onto glass every week just by following what’s on the label—ventilate, wear gloves if you’ve got sensitive skin, wipe and rinse well.
I see more benefits in teaching folks to check what’s in their cleaning closet and take small steps: store bottles out of kids’ reach, avoid mixing with other cleaners, and use with enough fresh air. For businesses, rotating staff and choosing milder products keeps people healthier without slowing down the job. If someone at home does have an allergic reaction, stop using and talk to a doctor before blaming the chemical—sometimes scents or dyes do more harm than the main ingredient.
Switching out DPGME for "natural" cleaners comes with its own trade-offs. Vinegar or lemon juice cleans well for many tasks, but might not tackle greasy buildup or dry quickly enough for certain jobs. Anyone sensitive to one product might need to experiment. Changing cleaning routines to use less product, keep things ventilated, and only buy strong chemicals when absolutely necessary protects families better than worrying about every ingredient list.
Dipropylene Glycol Butyl Ether pops up in places many folks wouldn’t expect: cleaning products, paints, cosmetics, and even inks. I remember walking through a factory and catching that faint, sweet smell – behind the scenes, solvents like this one do real work making products safer and easier to use. Its clear, colorless appearance gives no hint of its versatility or significance. Once you dig into the nitty-gritty of what goes on, the importance comes to light.
This chemical stands out because it manages to balance two worlds: it’s both water-soluble and oil-soluble. In practice, it blends easily into water-based and oil-based formulas. That means less worrying about separating layers in cleaning sprays or paint. Its boiling point sits around 230°C (446°F), so it handles high-heat environments without vanishing into thin air. I’ve dealt with other solvents that just evaporate too quickly, making work unpredictable or sticky.
It pours like a light syrup, not watery, not too thick either. The low odor helps a lot, especially in closed spaces or products that need to stay pleasant to the nose. Many folks use cleaning sprays every day, and harsh-smelling chemicals drive people away faster than anything. Dipropylene Glycol Butyl Ether gives a solution that’s easy to work with and doesn’t leave an offensive trail.
Its chemical backbone sits on ether groups—that means it plays well with many other substances. As a mid-range solvent, it dissolves greasy residues and stubborn dirt, so it finds a home in both household and industrial cleaning solutions. Its stability means chemical reactions don’t kick in by accident, which is a boon for manufacturers wanting products with decent shelf lives.
There’s also low flammability; I’ve read accident reports, and substances that catch fire at low temperatures can destroy a workday in seconds. Dipropylene Glycol Butyl Ether cuts that risk, so factories, janitors, and even artists can focus on the task at hand. Its vapor pressure keeps emissions low—less floating in the air, less breathing in unwanted fumes. For me, that detail touches on worker health. Not everything labeled ‘solvent’ is created equal, and some can irritate eyes, nose, and lungs. This one feels tame by comparison to more aggressive products of the past.
No chemical is without its hazards. It can cause skin and eye irritation with prolonged exposure, and in large doses it’s not something I’d want splashed on me or inhaled all day. Simple precautions make a difference—gloves, goggles, and decent ventilation. In the environmental picture, its low volatility makes it less likely to travel far, but waste treatment matters. Pouring this down the drain or dumping it in the open skips over the real problem of chemical buildup in water.
Weighing alternatives becomes part of the conversation for companies focused on greener chemistry. Less toxic substitutes sometimes get mixed results; it’s a balancing act—maintain performance, improve safety, avoid higher costs. Some newer solutions look promising, but in my experience, changing formulas sometimes leads to clogged sprayers or streaks on glass. It’s a puzzle that requires trial, error, and paying attention to what happens in real hands, not just on the drawing board.
Dipropylene Glycol Butyl Ether proves itself in all sorts of environments. Its properties offer reliability in performance, taste in safety, and flexibility in uses. People creating products or cleaning up after a spill don’t usually think about chemical makeups, but details like these affect how safely, efficiently, and comfortably a job gets done. Getting it right goes beyond checking a chemical list—it reaches into daily routines and even long-term health.
Dipropylene Glycol Butyl Ether doesn’t show up in most households, but it quietly powers plenty of industrial and commercial cleaning products. A lot of folks might never hear its name, though warehouses and smaller manufacturers know it well. The way people manage a large container of this liquid, compared to a tiny bottle of vinegar, couldn’t be more different. For starters, storage isn’t about just keeping shelves tidy. It’s about reducing risks, protecting workers, and making sure the neighborhood avoids a nasty surprise.
Firefighters and safety inspectors both hate surprises. This chemical isn’t highly flammable, but it catches fire with enough heat and an open flame. Keep big drums or barrels in a cool spot, away from any source of sparks or heat. Sunlight streaming onto metal drums in a poorly ventilated shed can spell trouble quickly in summer. If the place heats up fast and air doesn’t move, vapors might collect and put everyone at risk.
Shoving every jug onto the same bottom shelf might make sweeping easier, but those containers deserve more respect. You want spill trays under them, not just cardboard. Metal shelving stands up better to leaks, especially if somebody nervous tries to move a half-full container awkwardly. Vapors aren’t friendly, particularly in tight quarters—so always plan for proper airflow. Even cleaning crews notice the sharp, synthetic scent if the room feels stuffy.
Most lessons in safe handling come from experience—usually a mistake, a scare, or sometimes from the stories of an old shop manager. Gloves save hands from rough spots, and goggles keep stinging liquid out of eyes. Home brands may skip certain warnings, but industrial supplies often label their drums with big, bold statements for a reason. People trust what’s familiar, but the new guy in the warehouse might miss the hazard if nobody points it out.
Pumping the liquid out of larger containers should never mean improvising a siphon with a piece of garden hose. Specialized pumps and proper tools protect workers from accidental splashes and keep fumes where they belong. Setting up a job with enough absorbent material nearby saves headaches. In the event a container leaks or tips, you don’t want anyone scrambling to find rags.
Mixing or diluting can be the trickiest moment. Labels provide ratios, but rushing leads to spills, or worse, creating unexpected reactions if it contacts acids or incompatible cleaners. Never assume water alone solves every spill. Some chemicals mix in ways that make slippery floors or surprise fumes. Experience says double-check before adding anything directly into an unknown drum.
Many accidents owe more to shortcuts than to genuine ignorance. For every fancy label or lengthy safety sheet, a short training session often sticks better. Role-playing what to do during a spill, even just once, prepares everyone for a real emergency. Knowing where the nearest eyewash station sits, or which fire extinguisher can take care of a chemical fire, counts for more than a signed checklist.
In older shops or smaller businesses, reminders posted on walls or steps outlined in bold tape often shield against the day someone makes a rushed decision. Storing chemicals like Dipropylene Glycol Butyl Ether alongside clutter or unrelated supplies invites confusion. Clear labeling, distinct zones, and a bit of common sense can turn a cramped storage area into a safer workspace.
Getting storage and handling right doesn’t revolve around expensive equipment or endless paperwork. It comes down to habits—watching the details, protecting the workers, and planning for the accident nobody wants. Folks who take the time to teach these lessons, and actually enforce them, see fewer close calls and better days at work.
Walk into most hardware stores or poke around under your kitchen sink, you’ll spot cleaning products and paints that use Dipropylene Glycol Butyl Ether (DPGBE). Companies like it because it mixes well with water and doesn’t evaporate too quickly. I once spent a couple of summers repainting interiors, and those long afternoons taught me just how familiar this chemical becomes to anyone who works with sprays, degreasers, and coatings. Even the fresh“clean” smells after a deep clean can come from this stuff. It’s more common than folks think.
Every time DPGBE gets sprayed or poured, small amounts make it into the air. I remember headaches after days spent in freshly cleaned buildings—nobody blamed the air, but we probably should have. Short exposures in ventilated spaces don’t bother most people, but after many hours, some start swearing by open windows. Mild eye and throat irritation can creep up—especially for those with asthma or chemical sensitivities. The Material Safety Data Sheets (MSDS) say to avoid breathing it in, so there’s something to that old advice about fans and fresh air.
People working every day around solvents hold the real risk. The U.S. National Institutes of Health found that DPGBE isn’t classified as a known carcinogen, and normal use doesn’t set off alarms, but large doses or exposure over long periods bring up questions about liver and kidney burden. Too often, companies skip airing out rooms or forget to provide gloves, trusting the “low toxicity” label a little too much.
I remember helping out at a car detailing shop downtown—the runoff after a day’s work always ended up in nearby drains. DPGBE travels through municipal water, heading for treatment plants that don’t always manage to take it all out. According to the European Chemicals Agency, DPGBE breaks down slowly in nature, although bacteria in water and soil help finish the job. Still, small traces hang around, especially in busy cities. Fish and other aquatic life can face harmful effects from high concentrations, disrupting the delicate balance we rarely see beneath the surface.
Open windows work wonders. Even better, companies could move toward more plant-based cleaners that break down quickly and don’t leave persistent traces. For folks at home, reading labels and skipping heavy-duty cleaners unless necessary can keep air fresher and bodies healthier. I’ve seen some green cleaning businesses make a name for themselves by cutting out chemical solvents altogether—customers notice the difference.
On the regulation side, more honest reporting from manufacturers—clearer ingredient lists and guidance about where to use these products—would help. Pouring chemical-laced water down the sink stirs up bigger issues, so better city-run hazardous waste collection could keep waterways cleaner.
In the end, cleaner habits start with small steps. If we treat DPGBE and chemicals like it with a bit more respect—ventilating, protecting skin, and keeping them out of drains—we’ll notice the difference at home, on the job, and in the waterways trickling past downtown streets.
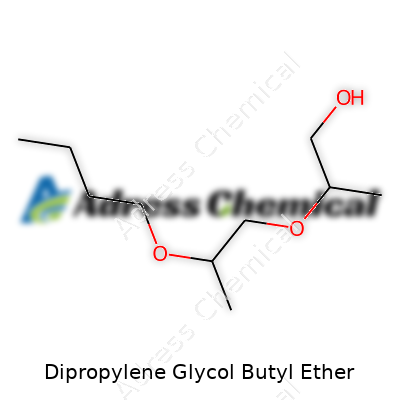
| Names | |
| Preferred IUPAC name | 3-(Butoxypropoxy)propan-1-ol |
| Other names |
DPnB Dipropylene glycol monobutyl ether Butoxypropanol 1-Butoxy-2-(2-propoxyethoxy)propane |
| Pronunciation | /daɪˈproʊpiːliːn ˈɡlaɪˌkɒl ˈbjuːtɪl ˈiːθər/ |
| Identifiers | |
| CAS Number | 29911-28-2 |
| 3D model (JSmol) | ``` /$3Dmol~{"mol":"C=CCCCOCC(C)OC(C)CO","format":"smi"} ``` |
| Beilstein Reference | 1728571 |
| ChEBI | CHEBI:81347 |
| ChEMBL | CHEMBL2206251 |
| ChemSpider | 156521 |
| DrugBank | DB14187 |
| ECHA InfoCard | 27-176-4 |
| EC Number | w/o EC Number |
| Gmelin Reference | 87832 |
| KEGG | C19668 |
| MeSH | D005876 |
| PubChem CID | 8183 |
| RTECS number | JM1575000 |
| UNII | Y98A03F44S |
| UN number | UN not regulated |
| Properties | |
| Chemical formula | C10H22O3 |
| Molar mass | 190.29 g/mol |
| Appearance | Clear, colorless liquid. |
| Odor | Mild glycol ether odor |
| Density | 0.95 g/cm³ |
| Solubility in water | miscible |
| log P | 0.84 |
| Vapor pressure | 0.01 mmHg @ 20°C |
| Acidity (pKa) | 13.5 |
| Basicity (pKb) | 7.43 |
| Magnetic susceptibility (χ) | -9.63×10⁻⁶ |
| Refractive index (nD) | 1.419 |
| Viscosity | 3.6 cP at 25°C |
| Dipole moment | 4.6 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.6 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -677.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6350 kJ/mol |
| Pharmacology | |
| ATC code | D07AX01 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | Warning, H319, H227 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P261, P264, P271, P280, P301+P312, P305+P351+P338, P337+P313, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | ≥ 93.5 °C |
| Autoignition temperature | 180°C |
| Explosive limits | 1.1% - 10.1% |
| Lethal dose or concentration | LD50 (oral, rat): 3300 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,118 mg/kg (rat, oral) |
| NIOSH | WI9775000 |
| PEL (Permissible) | 100 ppm |
| REL (Recommended) | 1.0% |
| Related compounds | |
| Related compounds |
Diethylene glycol butyl ether Propylene glycol butyl ether Tripropylene glycol monomethyl ether Ethylene glycol butyl ether Dipropylene glycol methyl ether |